Section 6 3 Periodic Trends
Explain why elements in the same group have similar properties.
Section 6 3 periodic trends. Learn vocabulary terms and more with flashcards games and other study tools. Start studying section 6 3. Circle the letter of the choice that best completes the statement or answers the question.
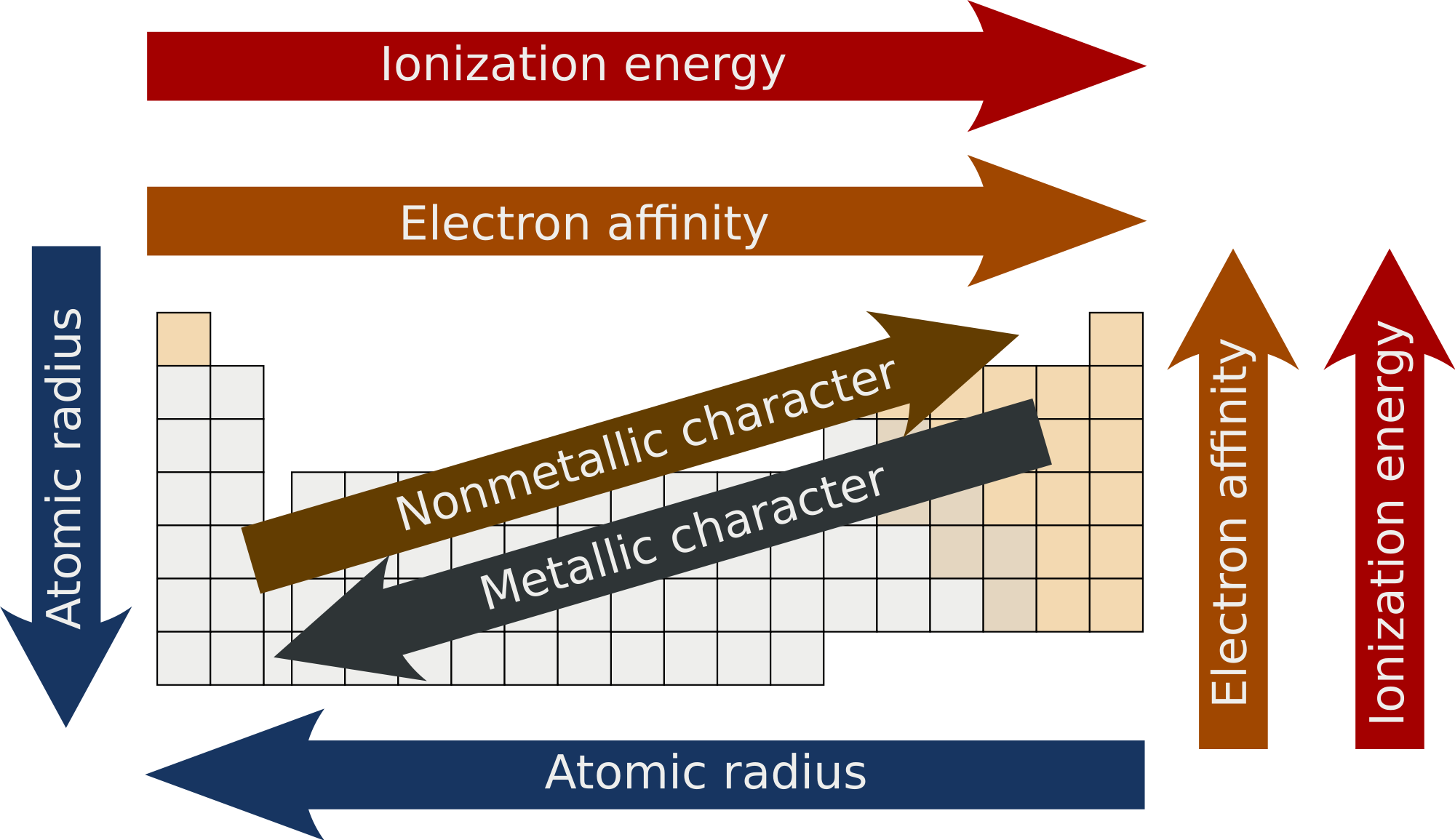
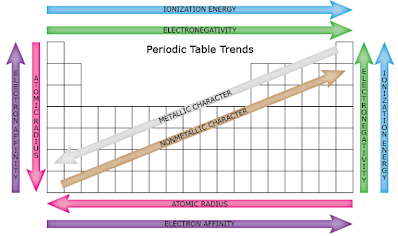
Ionization energy is the energy required to remove an electron from a gaseous atom. Section 6 3 periodic trends in your textbook read about atomic radius and ionic radius. Aod c 3 2 recognize periodic trends of elements including the number of valence electrons atomic size and reactivity.
Atomic radii cannot be measured directly because the electron cloud surrounding the nucleus does not have a clearly defined c. Look at the data for the alkali metals and noble gases. Neutrons protons and electrons may have formed within 10 4 second after the big bang and the lightest nuclei formed within 3 minutes.
A high ionization energy value indicates that the atom has a strong hold on its electrons and is not likely to lose an outer electron and form a positive ion. Section 6 3 periodic trends 171 group trends in atomic size in the figure 6 14 graph atomic radius is plotted versus atomic number. Chemistry 6 3 tips and tricks for balancing equations duration.
Section 6 3 periodic trends 3 sessions 11 2 blocks p ls 1. Identify the four blocks of the periodic table based on electron configuration. Prentice hall chemistry 2005 learn with flashcards games and more for free.
The atomic radius within these groups increases as the atomic number increases. Periodic trends section 6 3 objectives. Section 6 3 periodic trends 1.